what is the freezing point of ethanol Freezing ethanol aqueous
Today, I want to talk about the freezing point of ethanol, which is a topic of great interest in the scientific community. Ethanol, also known as ethyl alcohol, is a common ingredient in many alcoholic beverages and industrial products. It is a volatile liquid that is highly flammable and has a wide range of applications.
Freezing Point of Ethanol
Ethanol has a freezing point of -114.1 degrees Celsius or -173.4 degrees Fahrenheit. This means that ethanol will solidify and turn into a solid at temperatures below -114.1 degrees Celsius. The freezing point of ethanol is much lower than that of water, which freezes at 0 degrees Celsius or 32 degrees Fahrenheit. This is due to the difference in molecular structure between ethanol and water.
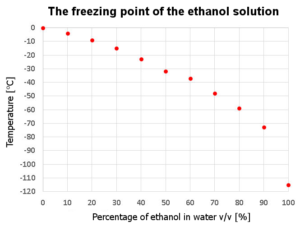 The image above depicts the freezing point of ethanol. As you can see, ethanol will freeze at extremely cold temperatures, making it a useful substance for various applications. However, it is important to handle ethanol with care, as it is highly flammable and can pose a safety risk if not handled properly.
The image above depicts the freezing point of ethanol. As you can see, ethanol will freeze at extremely cold temperatures, making it a useful substance for various applications. However, it is important to handle ethanol with care, as it is highly flammable and can pose a safety risk if not handled properly.
Freezing Point Diagram of Ethyl Alcohol-Water Mixture
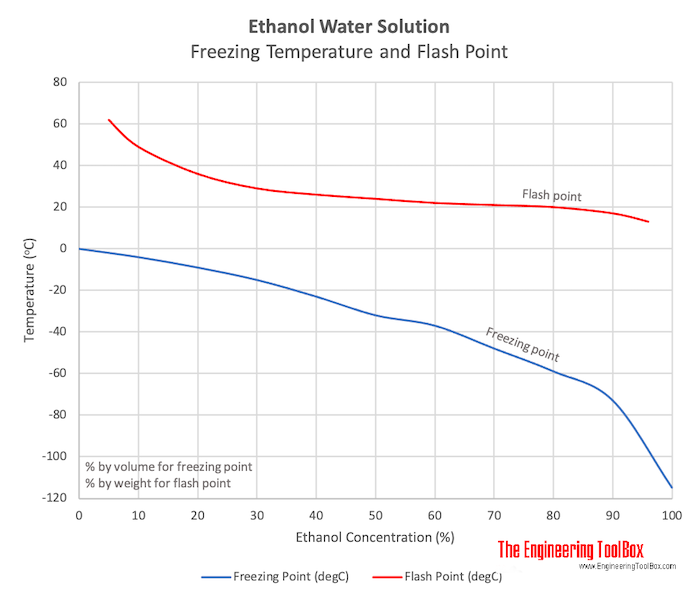
When ethanol is mixed with water, the freezing point changes. The image below shows a freezing point diagram of an ethyl alcohol-water mixture.
 As you can see from the diagram, the freezing point of the mixture decreases as the percentage of ethanol increases. This means that by adding ethanol to water, you can lower the freezing point of the solution. This property is utilized in various industries, such as antifreeze production, where a mixture of ethyl alcohol and water is used to prevent the freezing of liquids in cold temperatures.
As you can see from the diagram, the freezing point of the mixture decreases as the percentage of ethanol increases. This means that by adding ethanol to water, you can lower the freezing point of the solution. This property is utilized in various industries, such as antifreeze production, where a mixture of ethyl alcohol and water is used to prevent the freezing of liquids in cold temperatures.
In conclusion, ethanol has a very low freezing point of -114.1 degrees Celsius, making it a crucial substance in various applications. Understanding the freezing point of ethanol and its mixture with water is important for industries that rely on its properties. However, it is important to handle ethanol with caution due to its flammability. By studying the freezing point of ethanol, scientists and researchers can better understand its behavior and develop new applications for this versatile compound.
If you are looking for Ethanol Freeze Protected Water Solutions you’ve visit to the right web. We have 5 Pics about Ethanol Freeze Protected Water Solutions like What is the freezing point of ethanol? - ScienceNote.info, Freezing point of the ethanol aqueous solution. This figure is and also Freezing point diagram of ethanol/water solution. | Download Scientific. Here it is:
Ethanol Freeze Protected Water Solutions
 www.engineeringtoolbox.comethanol freezing
www.engineeringtoolbox.comethanol freezing
What Is The Freezing Point Of Ethanol? - ScienceNote.info
 sciencenote.infoethanol freezing solution depending percentage
sciencenote.infoethanol freezing solution depending percentage
Freezing Point Diagram Of An Ethyl Alcohol/water Mixture (values From
 www.researchgate.netfreezing ethyl values concentration
www.researchgate.netfreezing ethyl values concentration
Freezing Point Of The Ethanol Aqueous Solution. This Figure Is
 www.researchgate.netfreezing ethanol aqueous
www.researchgate.netfreezing ethanol aqueous
Freezing Point Diagram Of Ethanol/water Solution. | Download Scientific
 www.researchgate.netfreezing ethanol
www.researchgate.netfreezing ethanol
Freezing point diagram of an ethyl alcohol/water mixture (values from. Ethanol freezing. Ethanol freeze protected water solutions